Sarah E. Garcia, Nina E. Lillehei, and Eleza R. Valente, University of Denver
Nancy K. Grote, University of Washington
Benjamin L. Hankin, University of Illinois at Urbana-Champaign
Elysia Poggi Davis, University of Denver and University of California, Irvine

Abstract
Prenatal maternal depression affects both mother and fetus with long-term implications for offspring vulnerability to psychopathology through alterations to brain development, stress physiology, negative emotionality, and cognitive control. This article reviews evidence for the negative impact of prenatal maternal depression on offspring development and describes psychotherapeutic interventions that are used to mitigate prenatal maternal depression and thus, may positively impact infant and child development. The authors then describe an ongoing study to examine the effects on infant development of reducing prenatal maternal depression using a brief form of interpersonal psychotherapy adapted for use with pregnant women.
Maternal depression affects both mother and child and has significant long-term negative effects on infant development and child mental health risk (Goodman et al., 2011). The risks conferred by maternal depression experienced during the postpartum period have long been recognized; indeed mental health interventions have been developed to reduce postpartum maternal depression and, in turn, mitigate risk to child development. In contrast, the unique impact of depression experienced during the prenatal period on offspring development has only recently been understood. The potential positive effects that reducing prenatal depression could have on infant and child development are important to understand as they can set children on an early positive developmental trajectory. In addition, such interventions may be more economically efficient and more likely to reduce personal and societal burden than managing psychological symptoms and problematic behaviors after they arise. In this article, we review the deleterious effects of prenatal depression on fetal development as it relates to risk for later child psychopathology, describe an evidence-based psychosocial intervention for prenatal depression, and discuss how interventions to reduce maternal depression during pregnancy are uniquely situated to have cascading positive effects for both mother and child. Throughout the article we will use a composite case study of “Melissa,” a 24-year-old pregnant, Latina, single mother of a 5-year-old boy “Max,” to illustrate this idea.
Depression During Pregnancy
Depression is one of the most common prenatal complications. Studies suggest that rates of depression are as high or higher during pregnancy as compared to the postpartum period (Evans, Heron, Francomb, Oke, & Golding 2001). During pregnancy, an estimated 8.5 to 11% of women meet diagnostic criteria for major or minor depression (Gaynes et al., 2005), and even more women report elevated levels of prenatal depressive symptoms (Marcus, Flynn, Blow, & Barry, 2003). Rates of depression among high-risk samples of pregnant women, such as those exposed to contextual risk factors (e.g., poverty, racism, and violence), may be as high as 25% (Hobfoll, Ritter, Lavin, Hulsizer, & Cameron, 1995). Examples of psychosocial stressors experienced by pregnant women include lack of access to material resources, unfavorable employment conditions, heavy family and household responsibilities, low levels of social support, strain in intimate relationships, and medical complications. Some women, particularly those working lower status jobs, may find it difficult to attend prenatal appointments because of employer demands. The overlap of some depressive symptoms with pregnancy-related physical symptoms, such as sleep and appetite disturbances and fatigue, may be particularly burdensome for some women and may result in depressive symptoms being incorrectly attributed to pregnancy. Further, depressive symptoms are related to poor health behaviors in pregnant women including inadequate weight gain and substance use (Zuckerman et al., 1989), which further jeopardize the health of mother and child.
During a prenatal appointment in her first trimester, Melissa disclosed to her doctor that her current pregnancy was unplanned and she was experiencing conflict with, and receiving little support from, the father of her baby, who had wanted her to terminate the pregnancy. She reported depressed mood, excessive guilt, and crying spells, which she tried to hide from her son, and she had reduced interest in her usual activities, although she felt pressure to “put on a smile” for her son. She was unemployed and had missed recent job interviews as well as her prior prenatal appointment because she was struggling to get going and get out of the house in the morning. She was fatigued, spent much of the day napping, and struggled to make herself eat regular meals. Indeed, her doctor noted her failure to gain expected weight.
How Does Prenatal Depression Impact Offspring?
Development is more rapid during the prenatal period as compared to any other stage of life. The Fetal Programming and Developmental Origins of Disease models suggest that during periods of rapid fetal development or change, the fetus is particularly susceptible to environmental influences; these effects are thought to have persisting consequences for health and disease risk across the lifespan (Barker, 1998). In this way, prenatal depression may influence risk processes for subsequent psychopathology in offspring. Prenatal depression is linked to numerous, immediate adverse physical outcomes such as preterm birth (Fransson, Åden, Blennow, & Lagercrantz, 2011; 1.56 greater odds) and low birth weight (Grote et al., 2010), both of which have long-lasting implications for baby’s developmental course. In fact, the initial fetal programming studies illustrated that low birth weight predicted a range of physical and mental health outcomes including heart disease, diabetes, attention-deficit/hyperactivity disorder, and anxiety (Barker, Eriksson, Forsén, & Osmond, 2002; Class et al., 2014; Sømhovd, Hansen, Brok, Esbjørn, & Greisen, 2012). The consequences of prenatal depression on preterm birth and low birth weight appear to be greater for disadvantaged women (Alder, Fink, Bitzer, Hösli & Holzgreve, 2007).
Melissa experienced depression while pregnant with her son, Max. Max was born at 34 weeks gestation and he spent 2 weeks in the neonatal intensive care unit. She was very motivated to do what she could to carry her new baby to term, not feel incapacitated due to her depression, and avoid another neonatal intensive care unit stay for her new baby.
According to the Fetal Programming hypothesis, alterations to developing brain systems may be one of the key processes underlying the link between prenatal depression and offspring vulnerability to psychopathology. In addition, fetal exposure to maternal stressful negative life events (e.g., job loss, conflict with family), chronic psychosocial stress (e.g., experienced racism and homelessness), and stress hormones during the prenatal period continue to influence brain development, regulation of stress and emotion, and cognitive functioning throughout development and may contribute to the development of psychopathology (Huizink, Robles de Medina, Mulder, Visser, & Buitelaar 2003; Talge, Neal, Glover et al., 2007). It is important to note that these effects persist after considering postpartum influences (Bergman, Sarkar, O’Connor, Modi, & Glover, 2007). Prenatal depression may impact the offspring, and thus confer risk for later psychopathology, through four key pathways including alteration of brain development, physiological stress systems, negative emotionality, and cognitive control.
Brain Development
The exceptionally rapid pace of fetal brain development renders it susceptible to various influences. Neurogenesis, migration, axon and dendrite formation, as well as a multitude of other processes occur at an extraordinary pace throughout gestation. The brain of a full-term newborn has 100 billion neurons. Studies have revealed that prenatal depressive symptoms predict aspects of brain structure and function during infancy that pose risk for later psychopathology. For example, elevated prenatal depressive symptoms are associated with differences in amygdala functioning and structure as well as increased connectivity between prefrontal and limbic areas of the brain (Posner et al., 2016; Qiu et al., 2015). The impact of prenatal depressive symptoms on brain structure and function extends into childhood. Affected neural regions are related to greater child stress and emotional reactivity (Davis, Sandman, Buss, Wing, & Head, 2013; Sandman et al., 2018) and may contribute to risk for the development of psychopathology, particularly anxiety and depression (Lupien et al., 2011; Pérez-Edgar et al., 2007; Price & Drevets, 2010). For example, Sandman and colleagues (Sandman, Buss, Head, & Davis, 2015) found that prenatal depressive symptoms predicted decreased child cortical thickness at 6–9 years old, predominantly in frontal and temporal regions.
Stress Physiology
The hypothalamic-pituitary-adrenal (HPA) axis is one of the body’s primary stress regulatory systems and contributes to mental health. The prenatal environment is thought to affect the development of the offspring’s HPA axis (Davis, Glynn, Waffarn, & Sandman, 2011). Infants of mothers with prenatal depression demonstrate higher baseline cortisol concentrations (Field et al., 2004) and greater cortisol reactivity to stressors as compared to infants of mothers without prenatal depression (Brennan et al., 2008). Cortisol is a stress hormone that acts to mobilize the body’s resources to support the fight or flight response; short-term increases in cortisol are necessary to manage stress or challenge, but chronic elevations in cortisol may exert damaging effects. These effects of prenatal depression on dysregulated HPA-axis functioning, including a larger cortisol awakening response (Vänskä et al., 2016) and heightened cortisol reactivity in response to stress (Fernandes et al., 2014; Tollenaar, Beijers, Jansen, Riksen-Walraven, & de Weerth, 2011), continue into childhood (Guerry & Hastings, 2011; Lopez-Duran, Kovacs, & George, 2009).
Negative Emotionality
Negative emotionality is a risk factor for subsequent psychopathology, particularly anxiety and mood disorders (Ormel et al., 2013). Prenatal depressive symptoms predict greater infant and child negative emotionality over and above the effects of maternal depression during the postpartum period (Davis et al., 2007; Huot, Brennan, Stowe, Plotsky, & Walker, 2004; Rouse & Goodman, 2014). For example, prenatal depressive symptoms, particularly during the second trimester, predicted greater infant negative affectivity (Rouse & Goodman, 2014).

Development is more rapid during the prenatal period as compared to any other stage of life. Photo: VGstockstudio/shutterstock
Cognitive Control
Last, prenatal depression may impact offspring cognitive control, an aspect of executive functioning broadly related to general psychopathology (Snyder, Miyake, & Hankin, 2015). Prenatal depression predicted poor infant state regulation (Pacheco & Figueiredo, 2012) and toddler attentional and executive functioning problems (El Marroun et al., 2017; Van Batenburg-Eddes et al., 2013).
Offspring Psychopathology: Early Links With Prenatal Depression
Prospective studies demonstrate a link between prenatal depression, at diagnostic and subclinical levels, and offspring psychopathology outcomes, including anxiety, depression, and attention deficit/hyperactivity disorder later in adolescence and adulthood over and above the effects of postnatal maternal depression (Barker, Jaffee, Uher, & Maughan, 2011; Clements et al., 2015; Korhonen, Luoma, Salmelin, & Tamminen, 2012; Luoma et al., 2001). In a study of psychopathology outcomes in adult offspring, offspring exposed to maternal depression prenatally were 3.4 times more likely to meet criteria for major depressive disorder after accounting for postnatal maternal depression (Plant, Pariante, Sharp, & Pawlby, 2015).
When Max was a baby, Melissa had trouble soothing him when he began to cry, and her mother said he was a “fussy” baby. As Max grew up, she noticed that he often reacted to new situations or frustration by crying or having a temper tantrum. He had greater difficulty calming down when upset, compared to her cousins’ children. Max also had difficulty distracting himself after something upsetting had happened. His teachers noted that Max had difficulty following instructions, modulating his activity level to match his peers, and also had weeks when he became “moody” and withdrawn. Melissa wondered if his difficulties were related to the stress and depression she had experienced while pregnant with him. She worried that her new baby might have similar problems, which fueled her motivation to reduce her current levels of stress and depression.

Implementing depression screening early in pregnancy can have a multitude of cascading positive effects for both mother and child. Photo: Monkey Business Images/shutterstock
In summary, prenatal depression may exert effects on early fetal development that influence fetal brain and physiological stress reactivity systems. These effects may subsequently place the offspring at risk for a broad range of later psychopathology through intermediate risk mechanisms such as HPA-axis dysregulation, negative emotionality, and poor cognitive control that may be observed as early as infancy and toddlerhood. The evidence that the effects of prenatal depression persist after accounting for postpartum depression suggests the importance of looking to reduce maternal depression during the prenatal period in order to maximally decrease offspring risk for psychopathology. Further, the prevalence of prenatal depression and its unique contribution to offspring risk highlight the importance of addressing prenatal depression for professionals interested in child development and welfare.
Is Pregnancy an Optimal Time to Intervene on Maternal Depression?
During the prenatal period, women are better connected to the health care system because they have regular prenatal medical appointments, may be more receptive to guidance from a skilled professional (Battle, Salisbury, Schofield, & Ortiz-Hernandez, 2013; Dimidjian & Goodman, 2014), and may have more time relative to the postpartum period when they have a newborn for whom to care. Further, prenatal depression is a strong predictor of postpartum depression (O’Hara & Swain, 1996). For these reasons, the prenatal period provides a unique and opportune time to help women connect to mental health services.
Benefits of Prenatal Intervention for Mothers
Recommendations for prenatal care made by the American College of Obstetricians and Gynecologists include administration of a depression screener, for example the Edinburgh Postnatal Depression Screener (Cox, Holden, & Sagovsky, 1987), during the perinatal period. A few states (currently New Jersey, Illinois, and West Virginia) have adopted perinatal depression screenings as part of their health care policy. Such screenings put pregnancy health care providers in a unique position to be aware of their patients’ mental health and assist in connecting them to resources important for both mother and child (Rhodes & Segre, 2013). However, only West Virginia and Illinois specifically advocate screening during pregnancy. Implementing depression screening early in pregnancy can have a multitude of cascading positive effects for both mother and child. Having well-managed or remitted depression and established connections to mental health care benefits maternal mental health and fetal development and reduces the risk of postpartum depression (Grote et al., 2015). Establishing treatment during the prenatal period can help prepare the mother for the stressful and demanding postpartum period and ease adjustment to motherhood for first-time mothers (Collins, Dunkel-Schetter, Lobel, & Scrimshaw, 1993; Zlotnick, Johnson, Miller, Pearlstein, & Howard 2001). Sustained connection to a therapist throughout pregnancy and into the postpartum period allows for a quicker increase in support in response to resurgent depressive symptoms and socioeconomic stressors.
Despite the stressors Melissa experienced, she prioritized attending her prenatal appointments and was receptive to her midwife’s advice. Melissa’s midwife administered the Edinburgh Postnatal Depression Screener at her first prenatal visit and, based on her elevated score, discussed treatment options with her. Melissa was open to pursuing therapy because she was motivated to feel better and recognized that she would need greater support after the baby arrived.
Advantages of Prenatal Intervention for Child Outcomes
A small number of randomized control trials (RCTs) have shown that reducing a woman’s postpartum maternal depressive symptoms via intervention can impact her children’s psychopathological symptoms (for meta-analysis see Cuijpers, Weitz, Karyotaki, Garber, & Andersson, 2015). For example, in one RCT, psychotherapy for maternal depression showed reductions in child self-reported depressive symptoms at a 9-month follow-up (Swartz, Williamson, & Hariri, 2015).
Few research studies, to date, have tested the effect of reducing maternal depression prenatally on infant and child neuro-developmental outcomes (O’Connor, Monk, & Burke, 2016). Given the strong correlation between prenatal depression and a range of child developmental outcomes, intervention to reduce prenatal depression may be the most effective way to mitigate these early risks, including disrupted brain development, heightened stress reactivity, and negative emotionality, during a time of rapid development when these systems are most susceptible to environmental influences. Few studies have examined the effects of a psychotherapy intervention for prenatal depression on offspring psychological risk factors. As one example, 9-month-old infants of mothers whose prenatal depression was treated with cognitive–behavioral therapy evidenced less mother-reported reactivity to stress and negative affect compared to infants of mothers in the control group (Milgrom et al., 2015).
Interventions for Prenatal Maternal Depression
We focus on psychotherapy interventions for prenatal depression. Patients report enhanced preference for psychotherapy as compared to medication (Cuijpers, Cristea, Karyotaki, Reijnders, & Huibers, 2016). Also there is considerable debate regarding the safety of antidepressant medication usage during pregnancy for the developing fetus and later child outcomes (Udechuku, Nguyen, Hill, & Szego, 2010). There is a strong evidence base for the efficacy of psychotherapy for reducing prenatal depression (Grote et al., 2015; O’Mahen, Himle, Fedock, Henshaw, & Flynn, 2013; Spinelli, Endicott, & Goetz, 2016). In particular, adaptations of cognitive–behavioral therapy and interpersonal psychotherapy (IPT) have most often been tested. For example, evidence-based IPT that has been modified in order to engage and retain pregnant women of socioeconomically disadvantaged backgrounds in mental health treatment decreases depression during pregnancy relative to usual care (Grote et al., 2015).
Overview of Brief IPT and Its Application to Pregnant Women
Prenatal depression is strongly related to lack of social support, particularly from the spouse or partner (O’Hara & Swain 1996), which makes an interpersonal approach to the treatment of prenatal depression highly relevant. In fact, maternal social support during pregnancy has been linked to higher birth weight, positive birth outcomes, and child developmental outcomes (Feldman, Dunkel-Schetter, Sandman, & Wadhwa, 2000).
A brief version of IPT, consisting of 8 weekly individual sessions, has been developed (Swartz et al., 2004), and it received empirical support in a number of studies of pregnant women with depression (Grote et al., 2015; Swartz et al., 2004). It retains the core features of standard IPT, such as strengthening social supports, building on patient strengths and coping strategies, and resolving interpersonal problems. At the same time, brief IPT offers several advantages over standard IPT. First, it reduces the treatment burden for overwhelmed, pregnant, or parenting women with multiple acute and chronic stressors. Second, to promote a quicker treatment response, brief IPT techniques have been expanded to include behavioral activation strategies (Jacobson, Martell, & Dimidjian, 2006) that can be shared with family members or friends and assigned as weekly homework with an interpersonal focus. Treatment is focused on reducing symptoms and improving functioning in one of four possible interpersonal problem areas most closely linked with the onset of their depression. The problem areas are: a) Role Transition, a life upheaval such as geographic move or becoming physically ill; b) Role Dispute, a conflict with a significant other; c) Complicated Grief, the death of a loved one; or d) Complicated Pregnancy. Complicated Pregnancy addresses myriad difficulties women may experience navigating this life transition, including conflict with the father of the baby, medical issues related to the pregnancy, and unplanned nature of the pregnancy (Spinelli & Endicott, 2003).
Adaptations of MOMCare for Socioeconomically Disadvantaged Pregnant Women
MOMCare uses brief IPT to treat depression within a collaborative care model (Katon, 2003). The collaborative care model helps patients become active participants in their depression treatment, which is provided within the primary care setting by at least a masters-level mental health provider, supervised by a mental health team (e.g., can include an experienced IPT therapist and a prescribing medical doctor such as psychiatrist or obstetrician).
MOMCare has been enhanced to be culturally relevant to pregnant, economically disadvantaged, racially/ethnically diverse women with depression (Grote, Swartz, & Zuckoff, 2008). MOMCare’s cultural enhancements are designed to be relevant to the culture of race/ethnicity (Brown, Abe-Kim, & Barrio, 2003) and the culture of poverty (Belle, 2007). First, MOMCare includes a pre-treatment engagement session prior to beginning therapy (Grote, Zuckoff, Swartz, Bledsoe, & Geibel, 2007). The engagement session is designed (a) to address the woman’s ambivalence about, and commitment to, treatment and (b) to identify and attempt to resolve her unique practical, psychological, and cultural barriers to care, including finances, child care, transportation, scheduling problems, previous negative experiences with treatment, the stigma of depression, or fear of being misunderstood by a therapist of a different background. In the engagement session, psychoeducation about depression and treatment options are also provided. Respect for the woman’s cultural preferences regarding treatment options, her goals, cultural resources, and strengths are incorporated throughout treatment.
In addition, to address unique needs of economically disadvantaged women, a case management component is integrated into brief IPT to assist them in applying for jobs and in finding safe housing, food, and baby supplies. To increase treatment accessibility, therapy sessions can be conducted over the telephone. The therapist also engages in intensive outreach to retain patients in treatment, with telephone calls, texting, email, showing up at the patient’s non-stigmatizing public health setting, or meeting in coffee shops.
Melissa began treatment with a MOMCare therapist using brief IPT. She identified transportation and child care as significant barriers to treatment. She also shared that her mother had told her “being depressed is not something mothers have time for” and that these cultural views of depression as a mental weakness had prevented her from seeking treatment in the past. Melissa and her therapist decided to focus first on addressing her Complicated Pregnancy due to the unplanned nature of her pregnancy, little support from the father of the baby, and her financial stressors. Melissa and her therapist also worked on improving her communication with friends and family members who might be able to provide support during her pregnancy and as she prepared for birth.
Results from a randomized collaborative care trial of MOMCare showed that socioeconomically disadvantaged women with major depression, 58% of whom identified as a racial or ethnic minority, reported significantly greater improvement in depression severity and remission rates from before birth to 12 months postpartum, compared to intensive public health Maternity Support Services (Grote et al., 2015). Women with co-morbid posttraumatic stress disorder demonstrated the greatest improvements in depression (Grote et al., 2016). MOMCare also mitigated the risk of postpartum depressive symptoms and impaired functioning among women who experienced both prenatal depression and adverse birth events, such as preterm birth (Bhat et al., 2016), and was shown to be cost effective (Grote et al., 2017). In sum, findings from the MOMCare study suggest that a cost-effective collaborative care model reduces prenatal depression and could be integrated into obstetrics/gynecology clinics for pregnant women. Given the strengths of the MOMCare intervention model at reducing prenatal depression and addressing the unique challenges faced by disadvantaged pregnant women, MOMCare could be an optimal treatment to benefit both the mother and baby.
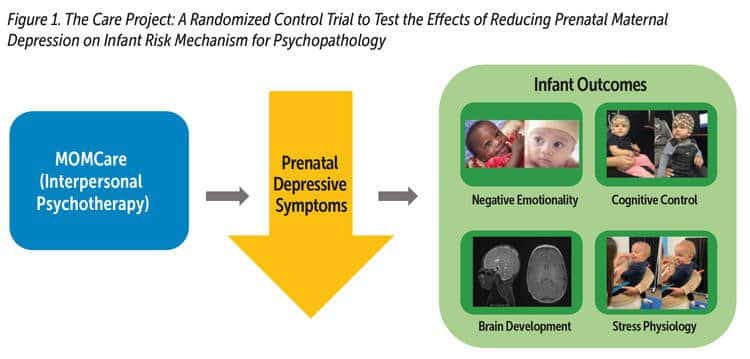
The Care Project: Does Reducing Prenatal Maternal Depression Improve Infant Outcomes?
Reducing postnatal maternal depression has positive effects on child development and may reduce risk for offspring psychopathology. Efficacious treatments exist to reduce prenatal depression (Grote et al., 2015; Spinelli et al., 2016). Yet at present, it is not known whether decreasing prenatal depression will positively impact fetal neurodevelopment and, in turn, mitigate infant risk for later psychopathology. Our current RCT, the Care Project, uses the MOMCare model to test whether reducing maternal depression during the prenatal period, a time of rapid fetal development, will reduce known risk factors in the offspring related to prenatal depression and, thus, reduce the likelihood that these offspring will develop subsequent psychopathology (Davis, Hankin, Swales, & Hoffmann, 2018). We are measuring maternal depression at numerous times beginning in pregnancy through 18 months postpartum and assessing infant risk mechanisms (see Figure 1). We measure infant risk mechanisms including structural brain development using MRI, stress reactivity via cortisol concentrations in infant hair and saliva, and negative emotionality and cognitive control from maternal report, electroencephalography, and standardized laboratory temperament tasks.
Summary and Conclusions
In sum, prenatal maternal depression has unique negative effects on infant and child development, including higher risk for offspring psychopathology, beyond the risk conferred by postpartum maternal depression. Intervening on prenatal depression has several unique advantages. Reducing prenatal depression may not only benefit maternal well-being but is an optimal window for promoting neurodevelopment of the offspring. Reducing prenatal depression may promote offspring adaptive brain development, HPA-axis regulation, and cognitive control, and may lessen negative emotionality. In addition, practical concerns make the prenatal period an opportune time to connect women to mental health services. However, additional research is needed to ascertain whether intervening on prenatal depression does in fact have the suggested positive effects on offspring mental health.
For women who initiate services during pregnancy, continued care at appropriate intervals maximizes the chance of maintaining remission of depressive symptoms across pregnancy and throughout the postpartum period. First-time mothers and mothers from high-risk backgrounds may be especially vulnerable to emergent stressors during the postpartum period. Sustained connections to mental health providers are likely to be more immediately effective than renegotiating barriers to care and reinitiating services at various points throughout the postpartum period. In sum, evidence-based intervention for prenatal depression, particularly culturally sensitive models designed to be effective for women at greatest risk for prenatal depression, have the potential to have a significant, positive, and long-lasting impact on both the mother and child.
About the Authors
Sarah E. Garcia, PhD, is a postdoctoral researcher at the University of Denver. She is interested in the early detection of risk for depression and anxiety in children and adolescents and the intergenerational transmission of internalizing psychopathology. She is also interested in how knowledge of differences in risk processes for internalizing disorders may allow better understanding of which treatments might work particularly well for certain individuals. She currently is a postdoctoral researcher and serves as a MOMCare therapist on the Care Project.
Nina E. Lillehei is a research assistant in the Neurodevelopment Research Project at the University of Denver. Nina graduated from the University of Denver with a bachelor of arts in psychology and sociology. She is interested in understanding intergenerational transmission of risk and resilience across families. She currently works as a recruiter and study coordinator for the Care Project.
Eleza R. Valente is a research assistant in the Neurodevelopment Research Lab at the University of Denver. She graduated from the University of Arizona with a bachelor of science in nutritional science and biochemistry. She has participated in clinical research with socially disadvantaged individuals and is interested in understanding how sleep influences physical and mental health. She currently works as a recruiter for the Care Project and is involved in assessment of biological processes in pregnant women.
Nancy K. Grote, PhD, MSW, is a research associate professor, emerita, at the School of Social Work, University of Washington. Dr. Grote has received national and local funding for adapting evidence-based models of depression care to be relevant both to the culture of poverty and to the culture of race/ethnicity and for disseminating culturally relevant, evidence-based treatments for perinatal depression in socioeconomically disadvantaged women in obstetrics/gynecology clinics, public health systems, and sites where pregnant and postpartum women addicted to drugs and alcohol receive treatment services. She is the creator of MOMCare and collaborator on the Care Project described in this report.
Benjamin L. Hankin, PhD, is the Kanfer Professor of Psychology at the University of Illinois. He is a developmental psychopathologist interested in understanding the etiology and trajectories of depression and related internalizing problems over the lifespan, and in conducting randomized clinical trials to reduce emotional distress and risk mechanisms in parents and youth. He currently is co-principal investigator of the Care Project.
Elysia Poggi Davis, PhD, is a professor of psychology at the University of Denver and is on the faculty in the Department of Psychiatry at the University of California, Irvine. Her program of research, supported by the National Institutes of Health, evaluates the way that biological and behavioral processes during the prenatal period influence developmental trajectories. Her research has demonstrated the prenatal maternal distress impacts neurodevelopment with consequences for mental health in the offspring. She currently is co-principal investigator of the Care Project.
Suggested Citation
Garcia, S. E., Lillehei, N. E., Valente, E. R., Grote, N. K., Hankin, B. L, & Davis, E. P. (2019). Does brief psychotherapy with distressed pregnant women benefit mother and baby? ZERO TO THREE Journal, 39(5), 23–32.
References
Alder, J., Fink, N., Bitzer, J., Hösli, I., & Holzgreve, W. (2007). Depression and anxiety during pregnancy: A risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. The Journal of Maternal-Fetal & Neonatal Medicine, 20(3), 189–209. Source link
Barker, D., Eriksson, J., Forsén, T., & Osmond, C. (2002). Fetal origins of adult disease: Strength of effects and biological basis. International Journal of Epidemiology, 31(6), 1235–1239. Source link
Barker, D. J. (1998). In-utero programming of chronic disease. Clinical Science (London, England: 1979), 95(2), 115–128. Source link.
Barker, E. D., Jaffee, S. R., Uher, R., & Maughan, B. (2011). The contribution of prenatal and postnatal maternal anxiety and depression to child maladjustment. Depression and Anxiety, 28(8), 696–702. Source link
Battle, C. L., Salisbury, A. L., Schofield, C. A., & Ortiz-Hernandez, S. (2013). Perinatal antidepressant use. Journal of Psychiatric Practice, 19(6), 443–453. Source link
Belle, D. (1990). Poverty and women’s mental health. American Psychologist, 45(3), 385–389. Source link
Bergman, K., Sarkar, P., O’Connor, T. G., Modi, N., & Glover, V. (2007). Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. Journal of the American Academy of Child & Adolescent Psychiatry, 46(11), 1454–1463.
Bhat, A., Grote, N. K., Russo, J., Lohr, M. J., Jung, H., Rouse, C. E., … & Katon, W. (2016). Collaborative care for perinatal depression among socioeconomically disadvantaged women: Adverse neonatal birth events and treatment response. Psychiatric Services, 68(1), 17–24.
Brennan, P. A., Pargas, R., Walker, E. F., Green, P., Jeffrey Newport, D., & Stowe, Z. (2008). Maternal depression and infant cortisol: Influences of timing, comorbidity and treatment. Journal of Child Psychology and Psychiatry, 49(10), 1099–1107. Source link
Brown, C., Abe-Kim, J. S., & Barrio, C. (2003). Depression in ethnically diverse women: Implications for treatment in primary care settings. Professional Psychology: Research and Practice, 34(1), 10–19. Source link
Class, Q. A., Abel, K. M., Khashan, A. S., Rickert, M. E., Dalman, C., Larsson, H., … D’Onofrio, B. M. (2014). Offspring psychopathology following preconception, prenatal, and postnatal maternal bereavement stress, Psychological Medicine, 44(1), 71–84.
Clements, C. C., Castro, V. M., Blumenthal, S. R., Rosenfield, H. R., Murphy, S. N., Fava, M., & Perlis, R. H. (2015). Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Molecular Psychiatry, 20(6), 727–734. Source link
Collins, N. L., Dunkel-Schetter, C., Lobel, M., & Scrimshaw, S. C. (1993). Social support in pregnancy: Psychosocial correlates of birth outcomes and postpartum depression. Journal of Personality and Social Psychology, 65(6), 1243–1258.
Cox, J. L., Holden, J. M., & Sagovsky, R. (1987). Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. The British Journal of Psychiatry, 150(6), 782–786.
Cuijpers, P., Cristea, I. A., Karyotaki, E., Reijnders, M., & Huibers, M. J. H. (2016). How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta-analytic update of the evidence. World Psychiatry: Official Journal of the World Psychiatric Association, 15(3), 245–258. Source link
Cuijpers, P., Weitz, E., Karyotaki, E., Garber, J., & Andersson, G. (2015). The effects of psychological treatment of maternal depression on children and parental functioning: A meta-analysis. European Child & Adolescent Psychiatry, 24(2), 237–245.
Davis, E. P., Glynn, L. M., Schetter, C. D., Hobel, C., Chicz-Demet, A., & Sandman, C. A. (2007). Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child & Adolescent Psychiatry, 46(6), 737–746. Source link
Davis, E. P., Glynn, L. M., Waffarn, F., & Sandman, C. A. (2011). Prenatal maternal stress programs infant stress regulation. Journal of Child Psychology and Psychiatry, 52(2), 119–129. Source link
Davis, E. P., Hankin, B. L., Swales, D. A., & Hoffman, M. C. (2018). An experimental test of the fetal programming hypothesis: Can we reduce child ontogenetic vulnerability to psychopathology by decreasing maternal depression? Development and Psychopathology, 30(3), 787–806. Source link
Davis, E. P., Sandman, C. A., Buss, C., Wing, D. A., & Head, K. (2013). Fetal glucocorticoid exposure is associated with preadolescent brain development. Biological Psychiatry, 74(9), 647–655. Source link
Dimidjian, S., & Goodman, S. H. (2014). Preferences and attitudes toward approaches to depression relapse/recurrence prevention among pregnant women. Behaviour Research and Therapy, 54, 7–11. Source link
El Marroun, H., White, T. J., Fernandez, G., Jaddoe, V. W., Verhulst, F. C., Stricker, B. H., & Tiemeier, H. (2017). Prenatal exposure to selective serotonin reuptake inhibitors and non-verbal cognitive functioning in childhood. Journal of Psychopharmacology, 31(3), 346–355. Source link
Evans, J., Heron, J., Francomb, H., Oke, S., & Golding, J. (2001). Cohort study of depressed mood during pregnancy and after childbirth. BMJ (Clinical Research Ed.), 323(7307), 257–260.
Feldman, P. J., Dunkel-Schetter, C., Sandman, C. A., & Wadhwa, P. D. (2000). Maternal social support predicts birth weight and fetal growth in human pregnancy. Psychosomatic Medicine, 62(5), 715–725.
Fernandes, M., Stein, A., Srinivasan, K., Menezes, G., Renton, M., Zani, J., & Ramchandani, P. G. (2014). Maternal depression and fetal responses to novel stimuli: Insights from a socio-economically disadvantaged Indian cohort. Journal of Developmental Origins of Health and Disease, 5(03), 178–182. Source link
Field, T., Diego, M., Dieter, J., Hernandez-Reif, M., Schanberg, S., Kuhn, C., … Bendell, D. (2004). Prenatal depression effects on the fetus and the newborn. Infant Behavior & Development, 27, 216–229. Source link
Fransson, P., Åden, U., Blennow, M., & Lagercrantz, H. (2011). The functional architecture of the infant brain as revealed by resting-state fMRI. Cerebral Cortex, 21(1), 145–154. Source link
Gaynes, B. N., Gavin, N., Meltzer-Brody, S., Lohr, K. N., Swinson, T., Gartlehner, G., … Miller, W. C. (2005). Perinatal depression: Prevalence, screening accuracy, and screening outcomes. Evidence Report/Technology Assessment (Summary), (119), 1–8.
Goodman, S. H., Rouse, M. H., Connell, A. M., Broth, M. R., Hall, C. M., & Heyward, D. (2011). Maternal depression and child psychopathology: A meta-analytic review. Clinical Child and Family Psychology Review, 14(1), 1–27. Source link
Grote, N. K., Bridge, J. A., Gavin, A. R., Melville, J. L., Iyengar, S., & Katon, W. J. (2010). A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Archives of General Psychiatry, 67(10), 1012–1024. Souce link
Grote, N. K., Katon, W. J., Russo, J., Lohr, M. J., Curran, M. C., Galvin, E., & Carson, K. (2016). A randomized trial of collaborative care for perinatal depression in socio-economically disadvantaged women: The impact of comorbid PTSD. Journal of Clinical Psychiatry, 72(11), 1527–1537.
Grote, N. K., Katon, W. J., Russo, J. E., Lohr, M. J., Curran, M., Galvin, E., & Carson, K. (2015). Collaborative care for perinatal depression in socioeconomically disadvantaged women: A randomized trial. Depression and Anxiety, 32(11), 821–834. Source link
Grote, N. K., Simon, G., Russo, J., Lohr, M. J., Carson, K., & Katon, W. J. (2017). Incremental cost-benefit analysis of MOMCare: Collaborative care for perinatal depression in economically disadvantaged women. Psychiatric Services, 68(11), 1164–1171.
Grote, N. K., Swartz, H. A., & Zuckoff, A. (2008). Enhancing interpersonal psychotherapy for mothers and expectant mothers on low incomes: Adaptations and additions. Journal of Contemporary Psychotherapy, 38(1), 23–33. Source link
Grote, N. K., Zuckoff, A., Swartz, H., Bledsoe, S. E., & Geibel, S. (2007). Engaging women who are depressed and economically disadvantaged in mental health treatment. Social Work, 52(4), 295–308.
Guerry, J. D., & Hastings, P. D. (2011). In search of HPA axis dysregulation in child and adolescent depression. Clinical Child and Family Psychology Review, 14(2), 135–160. Source link
Hobfoll, S. E., Ritter, C., Lavin, J., Hulsizer, M. R., & Cameron, R. P. (1995). Depression prevalence and incidence among inner-city pregnant and postpartum women. Journal of Consulting and Clinical Psychology, 63(3), 445–453.
Huizink, A. C., Robles de Medina, P. G., Mulder, E. J. H., Visser, G. H. A., & Buitelaar, J. K. (2003). Stress during pregnancy is associated with developmental outcome in infancy. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 44(6), 810–818.
Huot, R. L., Brennan, P. A., Stowe, Z. N., Plotsky, P. M., & Walker, E. F. (2004). Negative affect in offspring of depressed mothers is predicted by infant cortisol levels at 6 months and maternal depression during pregnancy, but not postpartum. Annals of the New York Academy of Sciences, 1032(1), 234–236. Source link
Jacobson, N. S., Martell, C. R., & Dimidjian, S. (2006). Behavioral activation treatment for depression: Returning to contextual roots. Clinical Psychology: Science and Practice, 8(3), 255–270. Source link
Katon, W. J. (2003). Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biological Psychiatry, 54(3), 216–226.
Korhonen, M., Luoma, I., Salmelin, R., & Tamminen, T. (2012). A longitudinal study of maternal prenatal, postnatal and concurrent depressive symptoms and adolescent well-being. Journal of Affective Disorders, 136(3), 680–692. Source link
Lopez-Duran, N. L., Kovacs, M., & George, C. J. (2009). Hypothalamic–pituitary–adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology, 34(9), 1272–1283. Source link
Luoma, I., Tamminen, T., Kaukonen, P., Laippala, P., Puura, K., Salmelin, R., & Almqvist, F. (2001). Longitudinal study of maternal depressive symptoms and child well-being. Journal of the American Academy of Child & Adolescent Psychiatry, 40(12), 1367–1374. Source link
Lupien, S. J., Parent, S., Evans, A. C., Tremblay, R. E., Zelazo, P. D., Corbo, V., … Seguin, J. R. (2011). Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proceedings of the National Academy of Sciences, 108(34), 14324–14329. Soure link
Marcus, S. M., Flynn, H. A., Blow, F. C., & Barry, K. L. (2003). Depressive symptoms among pregnant women screened in obstetrics settings. Journal of Women’s Health, 12(4), 373–380.
Marcus, S. M., Flynn, H. A., Blow, F. C., & Barry, K. L. (2003). Depressive symptoms among pregnant women screened in obstetrics settings. Journal of Women’s Health, 12(4), 373–380. https://doi.org/10.1089/154099903765448880
Milgrom, J., Holt, C., Holt, C. J., Ross, J., Ericksen, J., & Gemmill, A. W. (2015). Feasibility study and pilot randomised trial of an antenatal depression treatment with infant follow-up. Archives of Women’s Mental Health, 18(5), 717–730. https://doi.org/10.1007/S00737-015-0512-5
O’Connor, T. G., Monk, C., & Burke, A. S. (2016). Maternal affective illness in the perinatal period and child development: Findings on developmental timing, mechanisms, and intervention. Current Psychiatry Reports, 18(3), 24. https://doi.org/10.1007/S11920-016-0660-Y
O’Hara, M. W., & Swain, A. M. (1996). Rates and risk of postpartum depression—A meta-analysis. International Review of Psychiatry, 8(1), 37–54. https://doi.org/10.3109/09540269609037816
O’Mahen, H., Himle, J. A., Fedock, G., Henshaw, E., & Flynn, H. (2013). A pilot randomized controlled trial of cognitive behavioral therapy for perinatal depression adapted for women with low incomes. Depression and Anxiety, 30, 679–687. https://doi.org/10.1002/Da.22050
Ormel, J., Jeronimus, B. F., Kotov, R., Riese, H., Bos, E. H., Hankin, B., …Oldehinkel, A. J. (2013). Neuroticism and common mental disorders: Meaning and utility of a complex relationship. Clinical Psychology Review, 33(5), 686–697. Source link
Pacheco, A., & Figueiredo, B. (2012). Mother’s depression at childbirth does not contribute to the effects of antenatal depression on neonate’s behavioral development. Infant Behavior and Development, 35(3), 513–522. Source link
Pérez-Edgar, K., Roberson-Nay, R., Hardin, M. G., Poeth, K., Guyer, A. E., Nelson, E. E., … Ernst, M. (2007). Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage, 35(4), 1538–1546. Source link
Plant, D. T., Pariante, C. M., Sharp, D., & Pawlby, S. (2015). Maternal depression during pregnancy and offspring depression in adulthood: Role of child maltreatment. The British Journal of Psychiatry, 207(3), 213–220.
Posner, J., Cha, J., Roy, A. K., Peterson, B. S., Bansal, R., Gustafsson, H. C., … Monk, C. (2016). Alterations in amygdala–prefrontal circuits in infants exposed to prenatal maternal depression. Translational Psychiatry, 6(11), E935–E935. Source link
Price, J. L., & Drevets, W. C. (2010). Neurocircuitry of mood disorders. Neuropsychopharmacology, 35(1), 192–216. Source link
Qiu, A., Anh, T. T., Li, Y., Chen, H., Rifkin-Graboi, A., Broekman, B. F. P., … Meaney, M. J. (2015). Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Translational Psychiatry, 5(2), E508–E508. Source link
Rhodes, A. M., & Segre, L. S. (2013). Perinatal depression: A review of US legislation and law. Archives of Women’s Mental Health, 16(4), 259–270. Source link
Rouse, M. H., & Goodman, S. H. (2014). Perinatal depression influences on infant negative affectivity: Timing, severity, and co-morbid anxiety. Infant Behavior and Development, 37(4), 739–751. Source link
Sandman, C. A., Buss, C., Head, K., & Davis, E. P. (2015). Fetal exposure to maternal depressive symptoms is associated with cortical thickness in late childhood. Biological Psychiatry, 77(4), 324–334. Source link
Sandman, C. A., Curran, M. M., Davis, E. P., Glynn, L. M., Head, K., & Baram, T. Z. (2018). Cortical thinning and neuropsychiatric outcomes in children exposed to prenatal adversity: A role for placental CRH? American Journal of Psychiatry, 175(5), 471–479.
Snyder, H. R., Miyake, A., & Hankin, B. L. (2015). Advancing understanding of executive function impairments and psychopathology: Bridging the gap between clinical and cognitive approaches. Frontiers in Psychology, 6, 328. Source link
Sømhovd, M. J., Hansen, B. M., Brok, J., Esbjørn, B. H., & Greisen, G. (2012). Anxiety in adolescents born preterm or with very low birthweight: A meta-analysis of case-control studies. Developmental Medicine & Child Neurology, 54(11), 988–994. Source link
Spinelli, M. G., & Endicott, J. (2003). Controlled clinical trial of interpersonal psychotherapy versus parenting education program for depressed pregnant women. American Journal of Psychiatry, 160(3), 555–562.
Spinelli, M. G., Endicott, J., & Goetz, R. A. (2016). Interpersonal psychotherapy for depressed pregnant women: A reanalysis of baseline depression severity. Journal of Clinical Psychiatry, 77(4): 535–540.
Swartz, H. A., Frank, E., Shear, M. K., Thase, M. E., Fleming, M. A. D., & Scott, J. (2004). A pilot study of brief interpersonal psychotherapy for depression among women. Psychiatric Services, 55(4), 448–450. Source link
Swartz, J. R., Williamson, D. E., & Hariri, A. R. (2015). Developmental change in amygdala reactivity during adolescence: Effects of family history of depression and stressful life events. American Journal of Psychiatry, 172(3), 276–283. Source link
Talge, N. M., Neal, C., Glover, V., & Early Stress, Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolescent Mental Health. (2007). Antenatal maternal stress and longterm effects on child neurodevelopment: How and why? Journal of Child Psychology and Psychiatry, 48(3–4), 245–261.
Tollenaar, M. S., Beijers, R., Jansen, J., Riksen-Walraven, J. M. A., & De Weerth, C. (2011). Maternal prenatal stress and cortisol reactivity to stressors in human infants. Stress, 14(1), 53–65. Source link
Udechuku, A., Nguyen, T., Hill, R., & Szego, K. (2010). Antidepressants in pregnancy: A systematic review. The Australian and New Zealand Journal of Psychiatry, 44(11), 978–996. Source link
Van Batenburg-Eddes, T., Brion, M. J., Henrichs, J., Jaddoe, V. W. V., Hofman, A., Verhulst, F. C., … Tiemeier, H. (2013). Parental depressive and anxiety symptoms during pregnancy and attention problems in children: A crosscohort consistency study. Journal of Child Psychology and Psychiatry, 54(5), 591–600. Source link
Vänskä, M., Punamäki, R.-L., Lindblom, J., Tolvanen, A., Flykt, M., Unkila-Kallio, L., … Tiitinen, A. (2016). Timing of early maternal mental health and child cortisol regulation. Infant and Child Development, 25(6), 461–483. Source link
Zlotnick, C., Johnson, S. L., Miller, I. W., Pearlstein, T., & Howard, M. (2001). Postpartum depression in women receiving public assistance: Pilot study of an interpersonal therapy-oriented group intervention. American Journal of Psychiatry, 158(4), 638–640. Source link
Zuckerman, B., Frank, D. A., Hingson, R., Amaro, H., Levenson, S. M., Kayne, H., … Bauchner, H. (1989). Effects of maternal marijuana and cocaine use on fetal growth. New England Journal of Medicine, 320(12), 762–768. Source link



